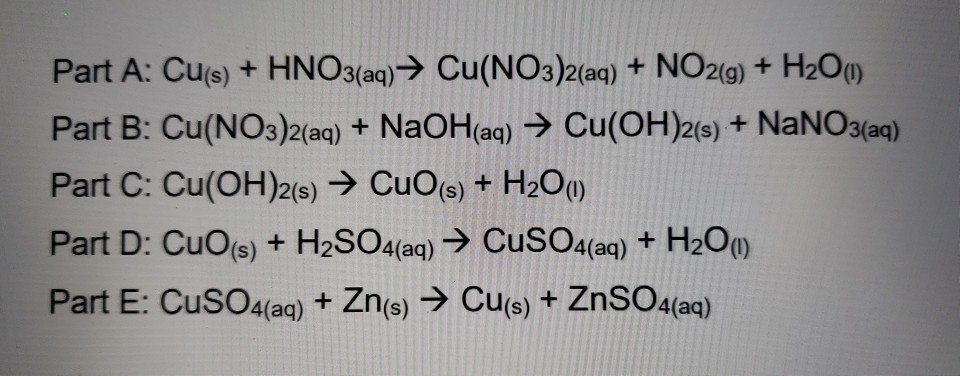
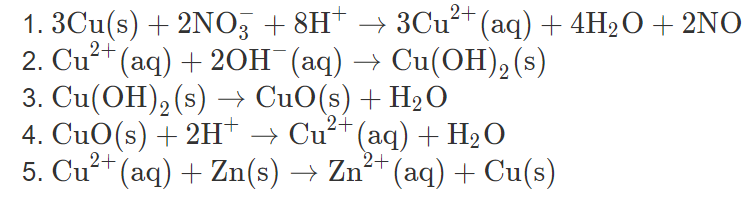Lab 21Date: Cupric Oxide from Cupric Sulfate Purpose Compare the experimental and calculated amounts of CuO produced in a rxn. Background CuSO 4 (aq) + - ppt download

Pushing the Limits of Rapid Anodic Growth of CuO/Cu(OH)2 Nanoneedles on Cu for the Methanol Oxidation Reaction: Anodization pH Is the Game Changer | ACS Applied Energy Materials

Temperature contour with CuO-H2O (DI) as a working fluid in HCE at 40 LPH. | Download Scientific Diagram
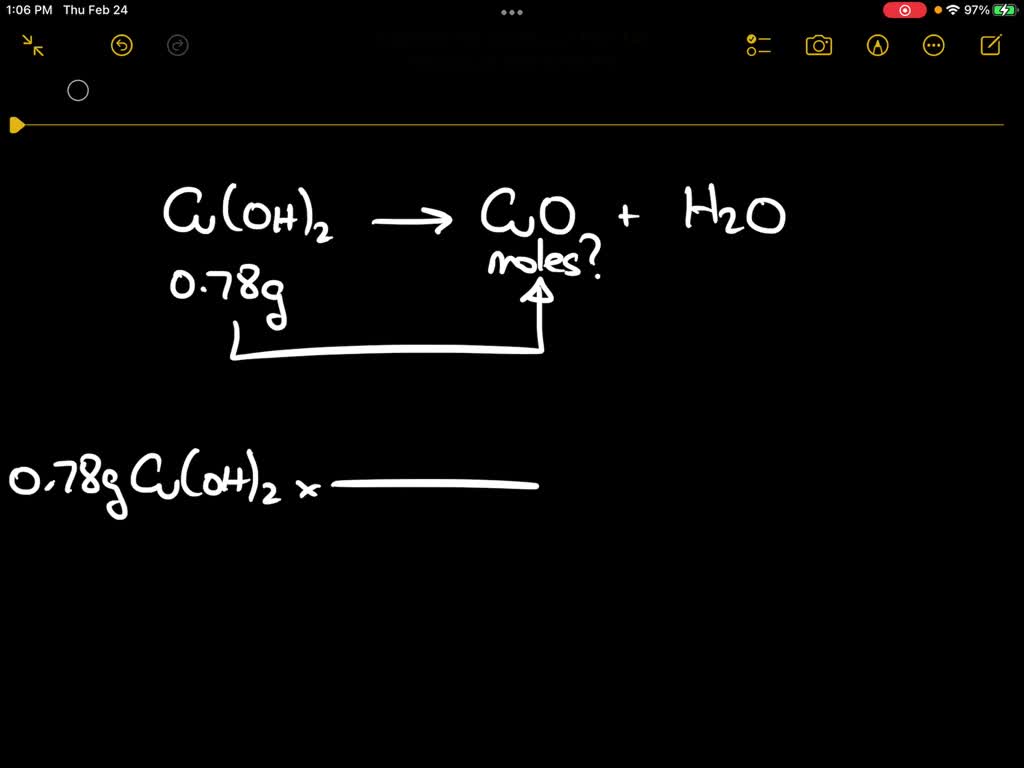
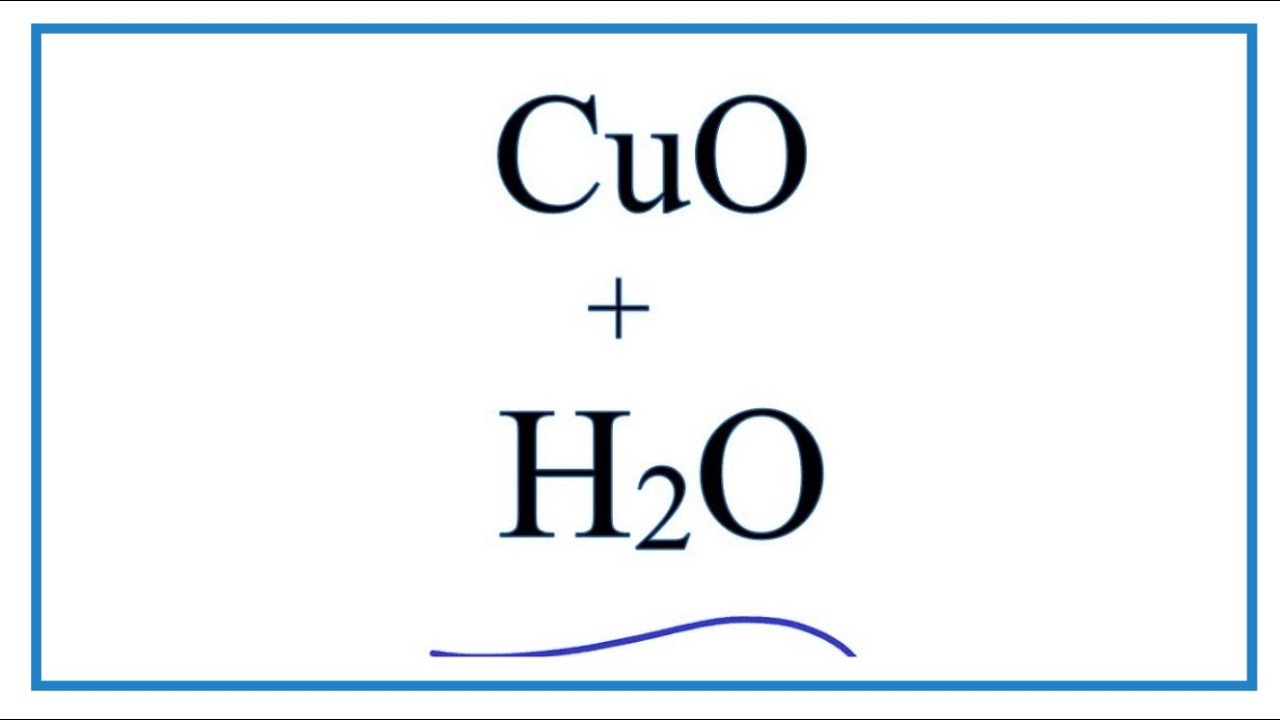
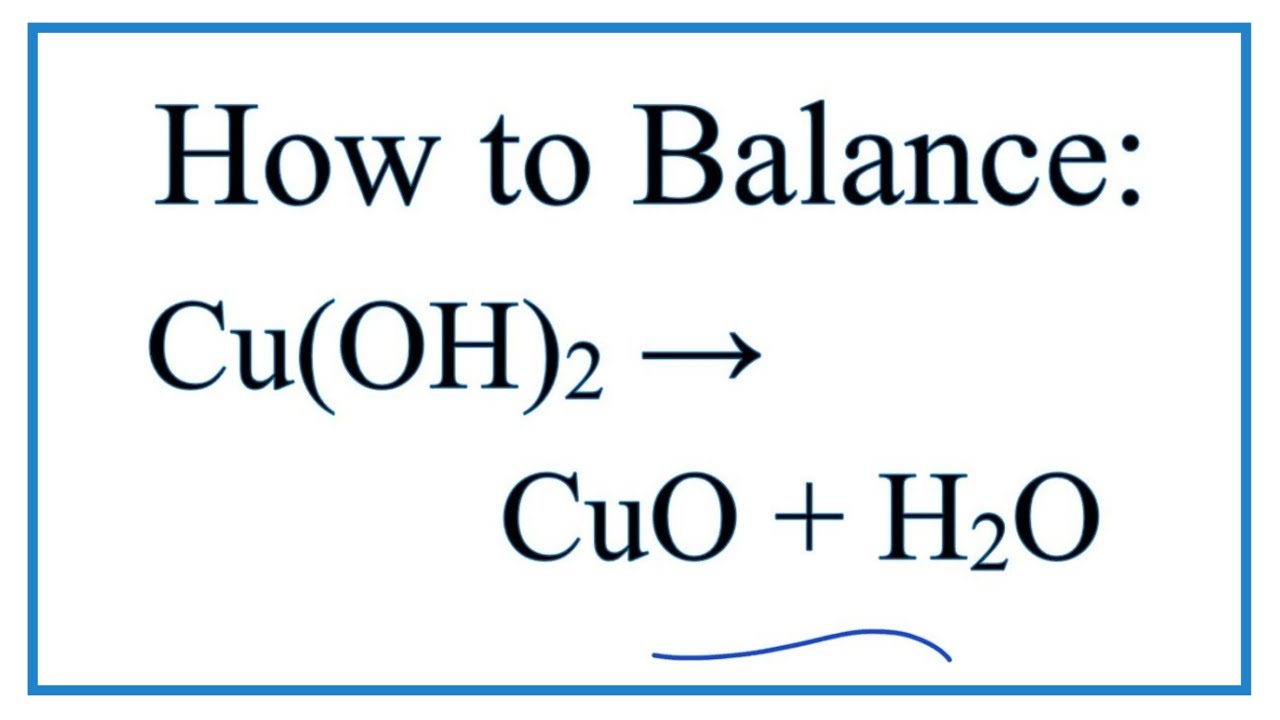
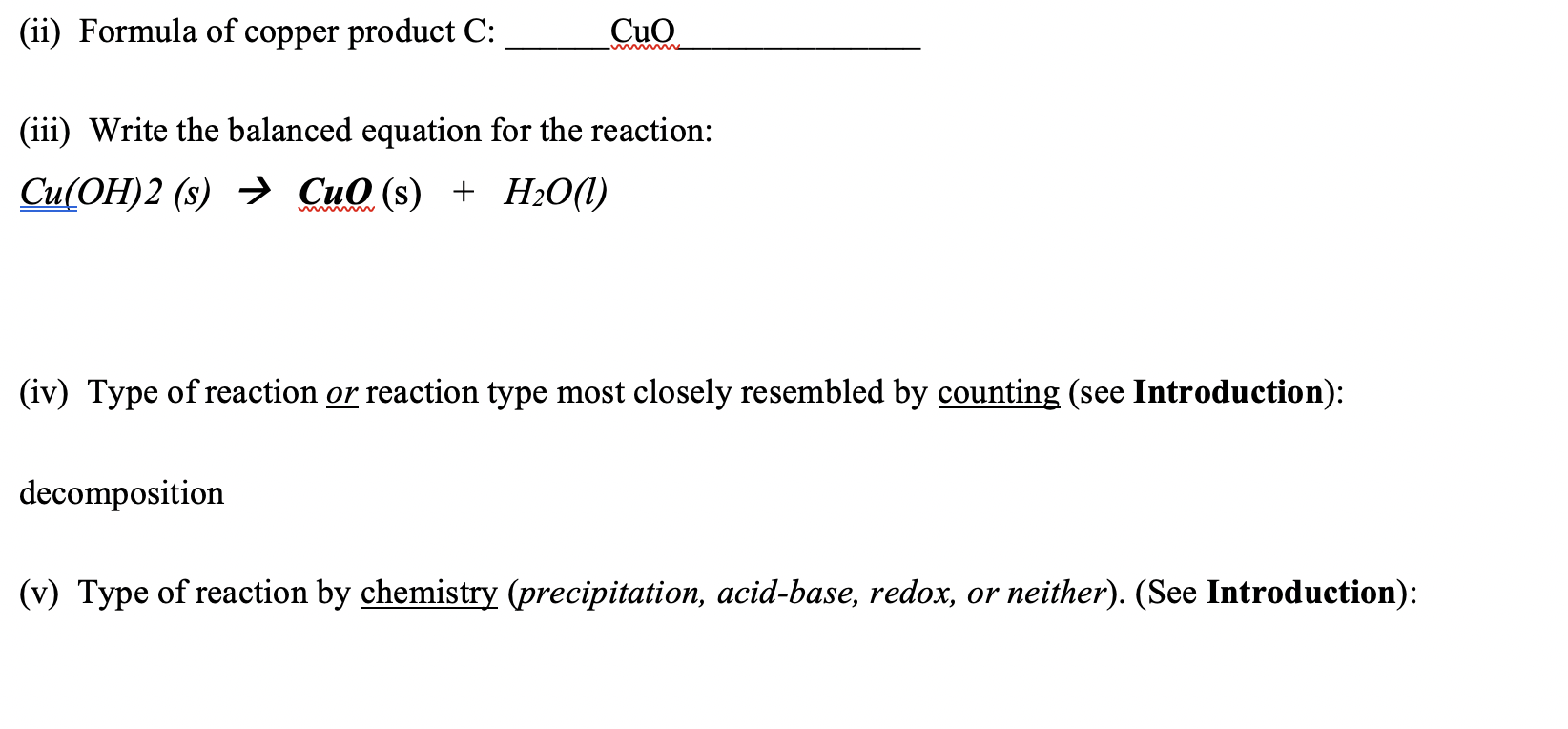
SOLVED: Upon heating Cu(OH)2 decomposes into CuO and H2O as shown below. Cu(OH)2 → CuO + H2O If 0.78 g of Cu(OH)2 was decomposed, calculate the moles of CuO produced Use 29Cu63.5,

Given the redox reaction: CuO (s) + H2(g)harr Cu(s) + H2O(g) i) Identify the species which undergo reduction and which undergo oxidation