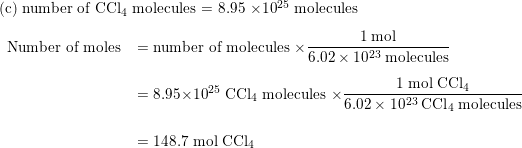Chem Notes Mole- SI unit for amount of matter Mole- SI unit for amount of matter mol 6.02 X representative particles= Avogadro's Number. - ppt download

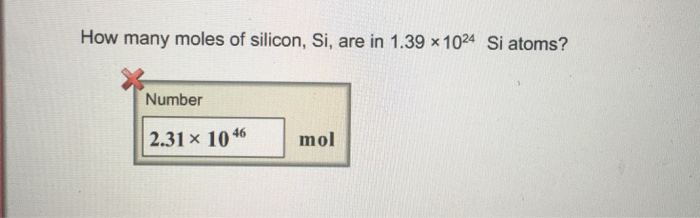
SOLVED: Perform the following conversions a. 1.51 X 1015 atoms of Si to mol of Si b. 4.25 X 10-2 mol of H2SO4 to molecules of HzSO4 c: 8.95 X 1025 molecules

Chapter 7 Chemical Quantities. The Mole SI base unit to measure the amount of a substance 1 mole of anything = x representative particles. - ppt download

SOLVED: Help me to determine Determine the mass, in grams, of 0.750 moles of Si (1 mol of Si has a mass of 28.09 g).