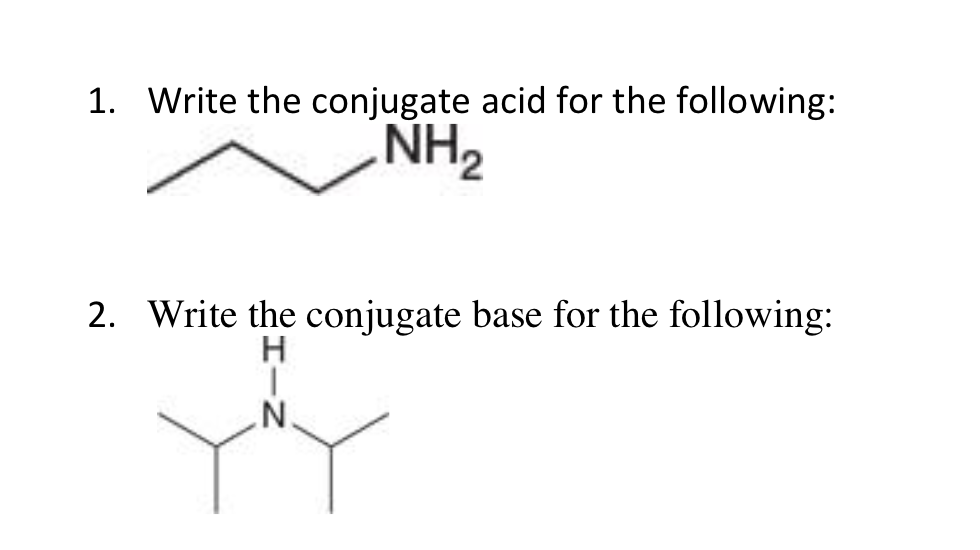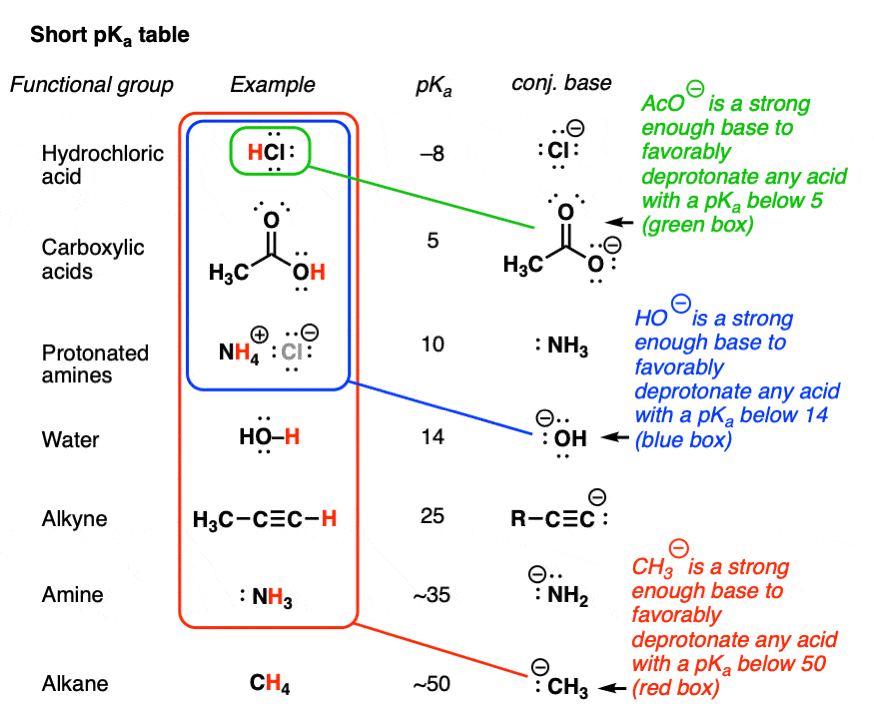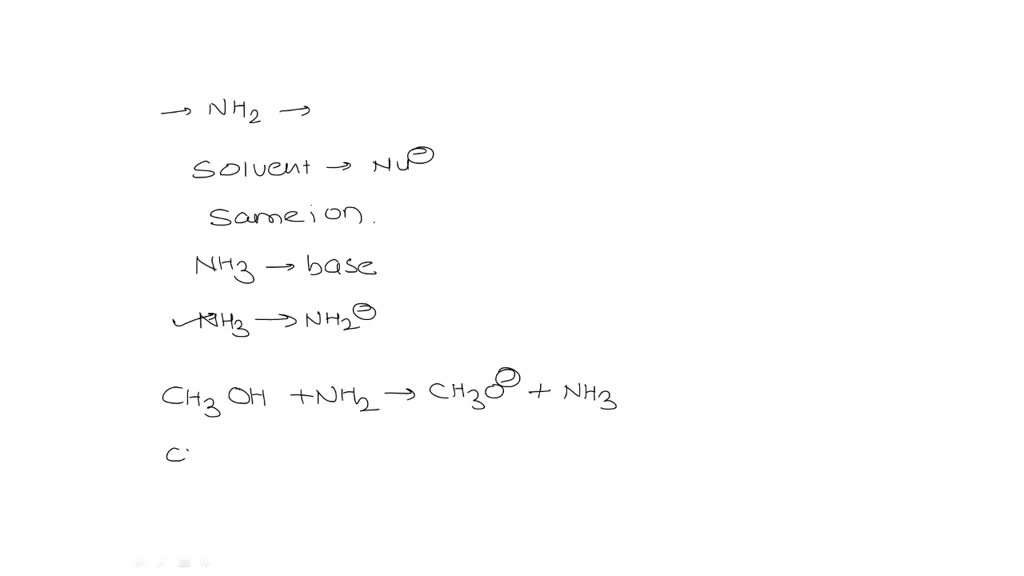
SOLVED: I already know the answer - please explain why I have no idea what this is talking about. The amide ion NH2- is a base which can only be used in
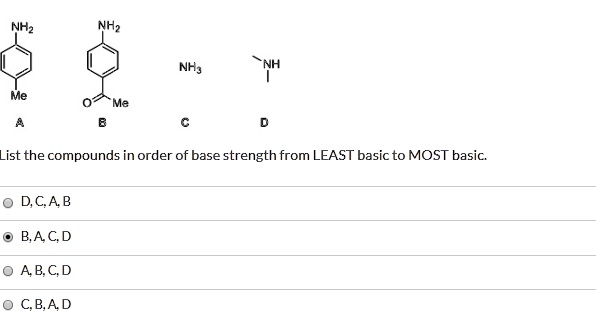
SOLVED: NH2 NH3 Me ist the compounds in order of base strength from LEAST basic to MOST basic: D,CA B BA C D ABC D CBA D

Ch2oh ch2 ch2 nh2 what is the nature acidic basic of this amino acid what can be the possible components present in the amino group so thai it is identifiable in a
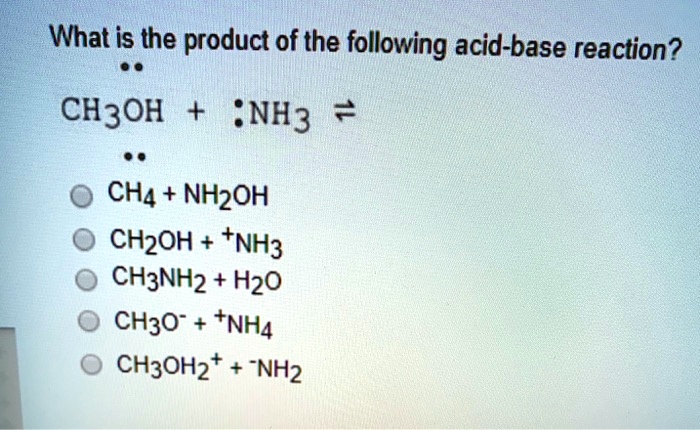
SOLVED: What is the product of the following acid-base reaction? CH3OH :NH3 2 CHA - NHZOH CH2OH +NH3 CH3NH2 Hzo CH3O" +NHA CH3OH2" NH2



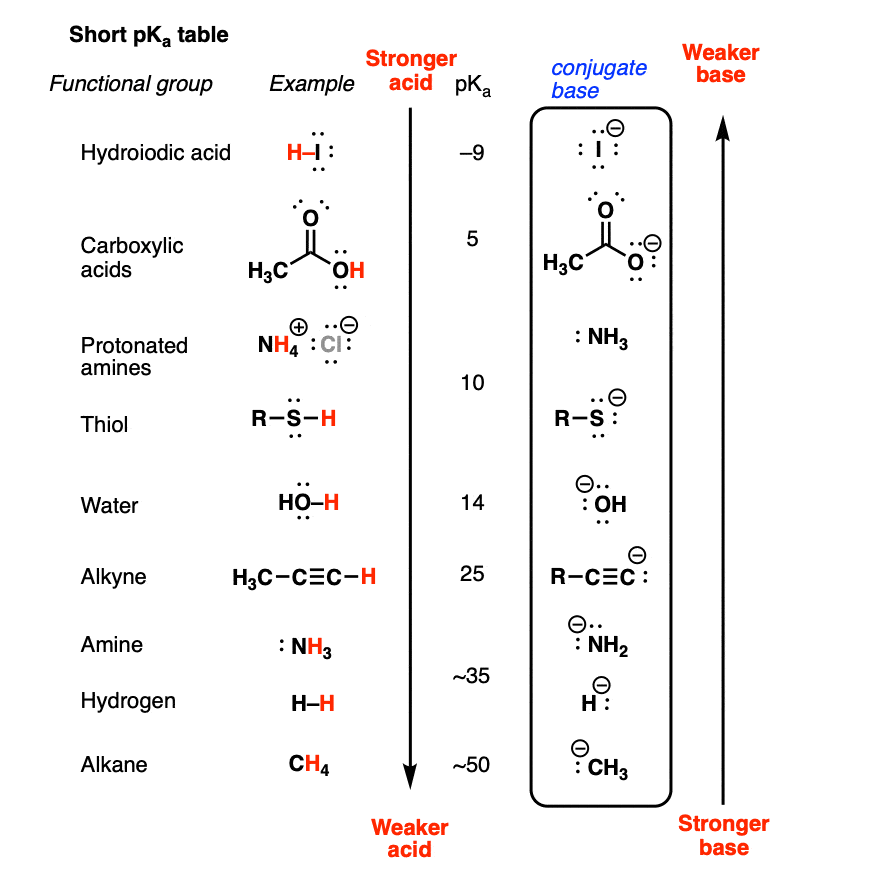

![Which is the correct for the following reaction: B (OH)3 + H2O → [B(OH)4]^ - + H^ + Which is the correct for the following reaction: B (OH)3 + H2O → [B(OH)4]^ - + H^ +](https://dwes9vv9u0550.cloudfront.net/images/5674386/ee9fa7d9-c7ef-4a7f-90dd-f3bb61402c59.jpg)