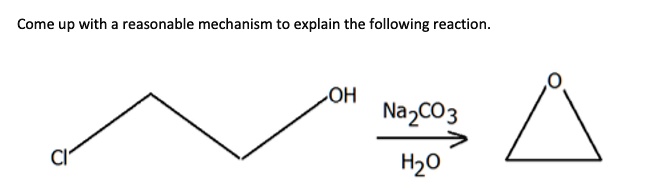
Scheme 3. Reagents and conditions: a) H2O, Na2CO3, rt, 1 min; b) CH3CN,... | Download Scientific Diagram

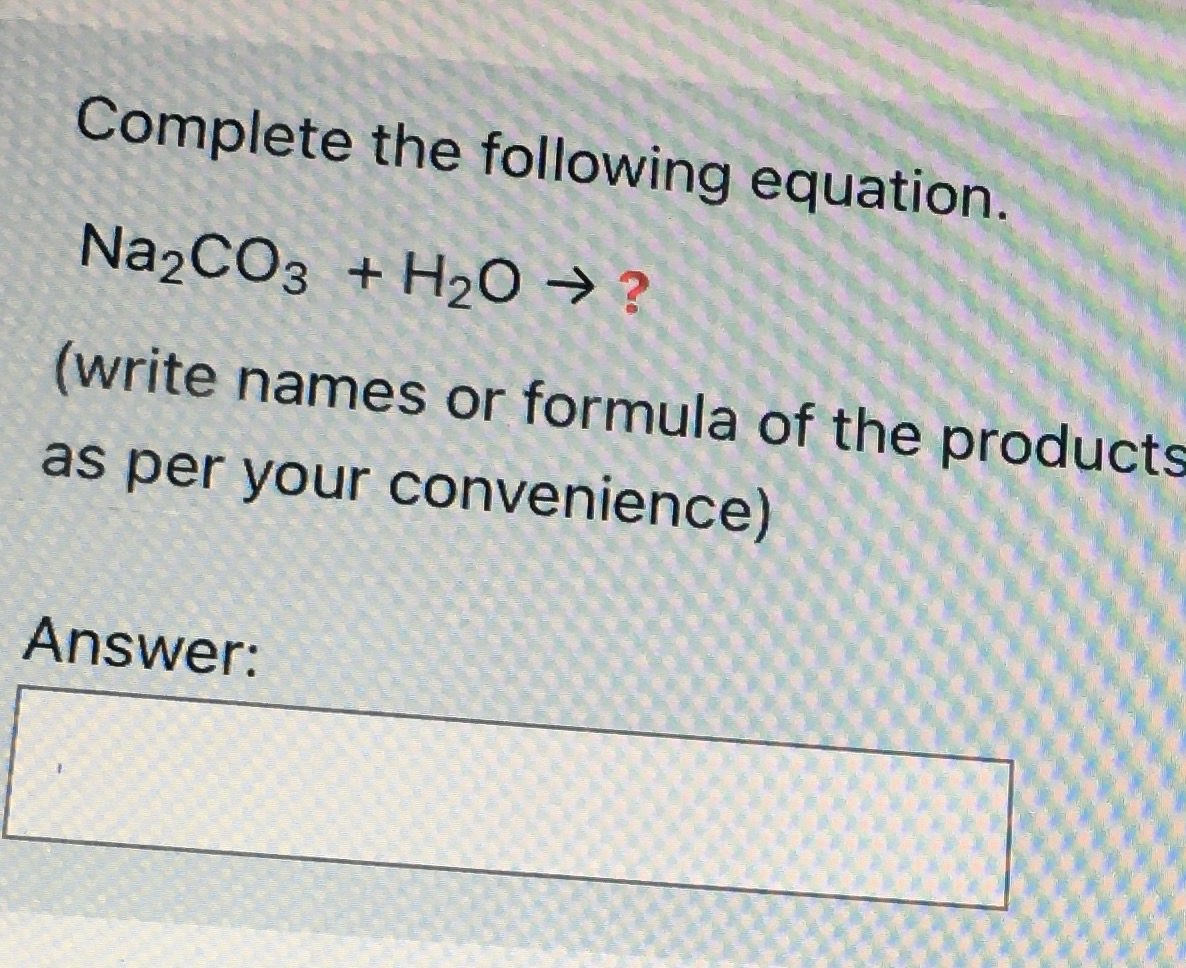
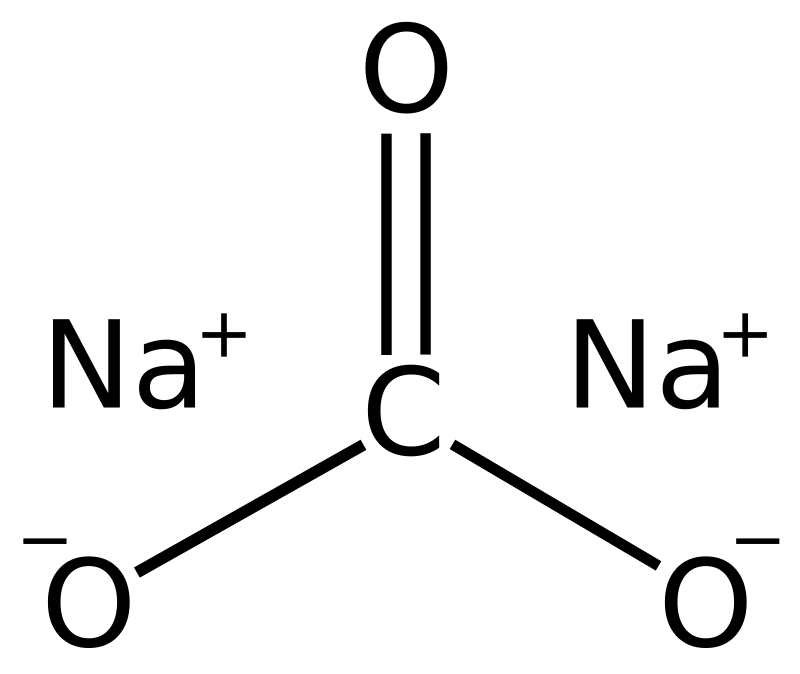
How to Balance Na2CO3+H2O→NaOH+CO2 | How to Balance Na2CO3+H2O→NaOH+CO2 | By Organic Chemistry Tutorial/Inorganic Chemistry/Science | Facebook
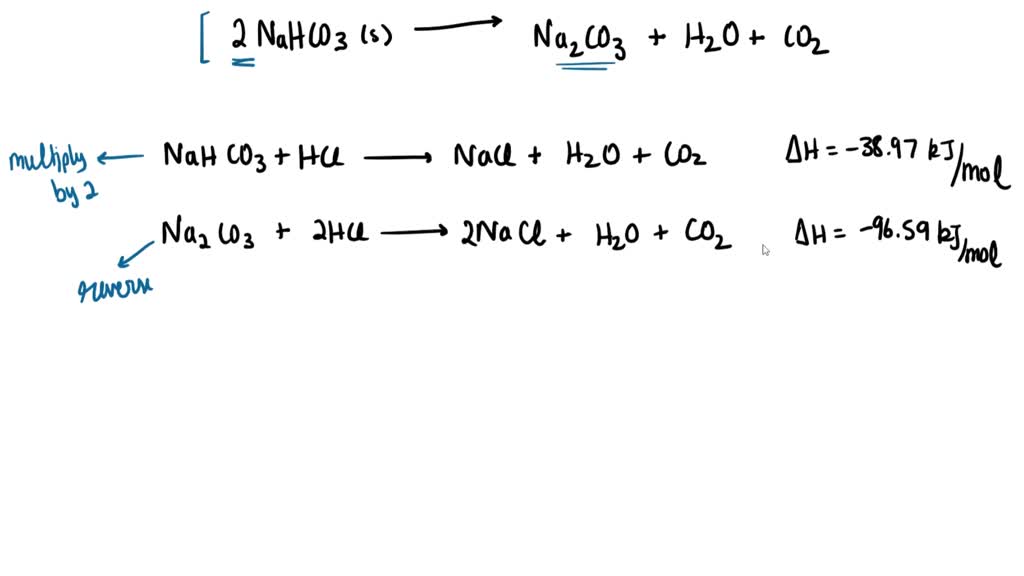
SOLVED: The following data are needed for this question. NaHCO3 (s) + HCl (aq) + NaCl (aq) + H20 (1) + CO2 (g) AH=-38.97 kJ mol-1 Na2CO3 (s) + 2HCl (aq) +
24. What is the gram equivalent mass of Na2CO3 in a) Na2CO3 + 2HCl= 2NaCl + H2O b) Na2CO3 + HCl = Nacl + NaHCO3
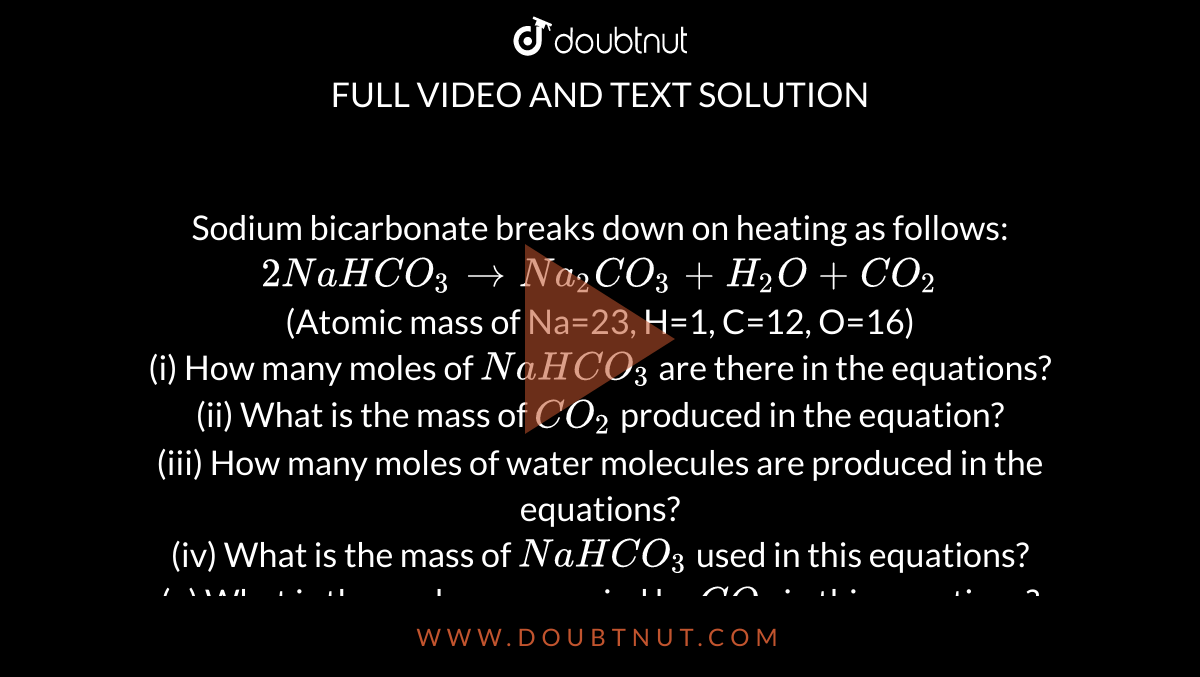
Sodium bicarbonate breaks down on heating as follows: 2NaHCO(3) to Na(2)CO(3)+H(2)O+CO(2) (Atomic mass of Na=23, H=1, C=12, O=16) (i) How many moles of NaHCO(3) are there in the equations? (ii) What is

Carbodiimide‐Driven Dimerization and Self‐Assembly of Artificial, Ribose‐Based Amphiphiles - Sun - 2022 - Chemistry – A European Journal - Wiley Online Library