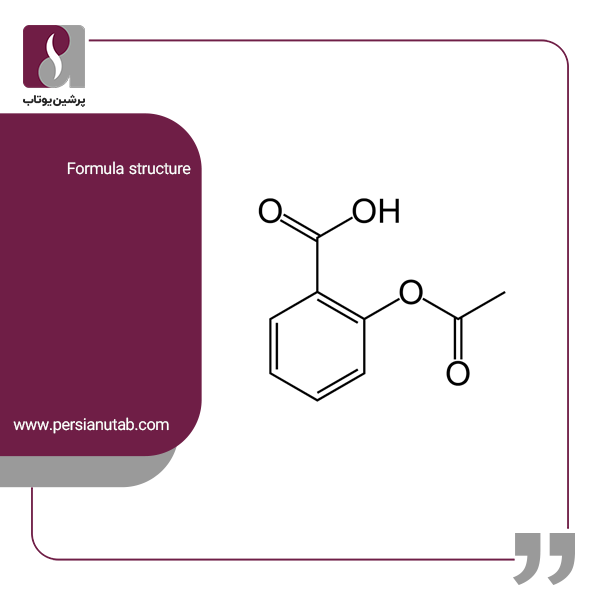
Potassium hydroxide, caustic potash, lye molecule. KOH is strong caustic base and alkali, ionic compound. Structural chemical formula and molecule mod Stock Vector Image & Art - Alamy
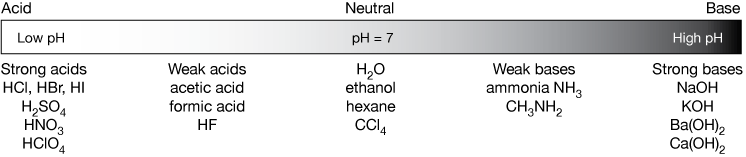
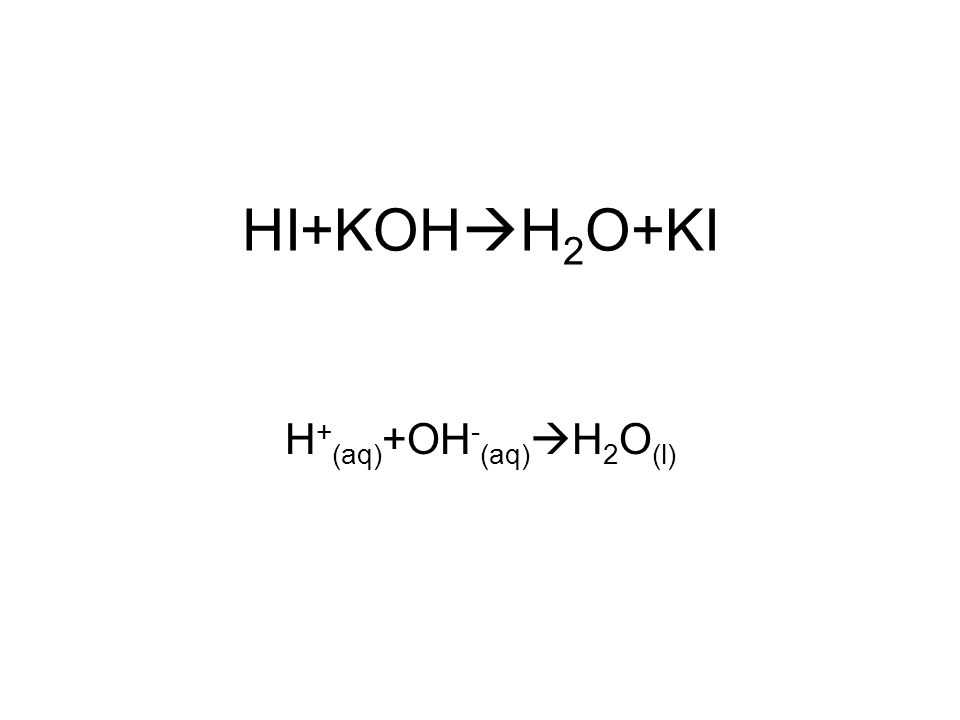
SOLVED: List the conjugate acid or conjugate base for each chemical. a. The acid HF b. The base KOH c. The base NH3 d. The acid HNO3 e. The acid HCOOH f.

Selective Focus of a Bottle of Pure Potassium Hydroxide or KOH Chemical Compound beside a Petri Dish with White Solid Pellets. Stock Photo - Image of flakes, base: 199192488