See: hydrogen reacts with oxygen to form water (H2O) according to the following equation: 2H2 + O2 → 2H2O - Brainly.com

n mole each of H2O, H2 and O2 are mixed at a suitable high temperature to attain the equilibrium 2H2O 2H2 + O2 . If y mole of H2O are dissociated and

calculate the equilibrium constant of H2 + O2 gives us H2O + CEO at 13957 if the equilibrium constant 135 - Chemistry - Equilibrium - 13886927 | Meritnation.com
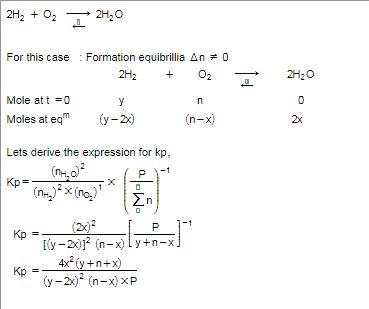
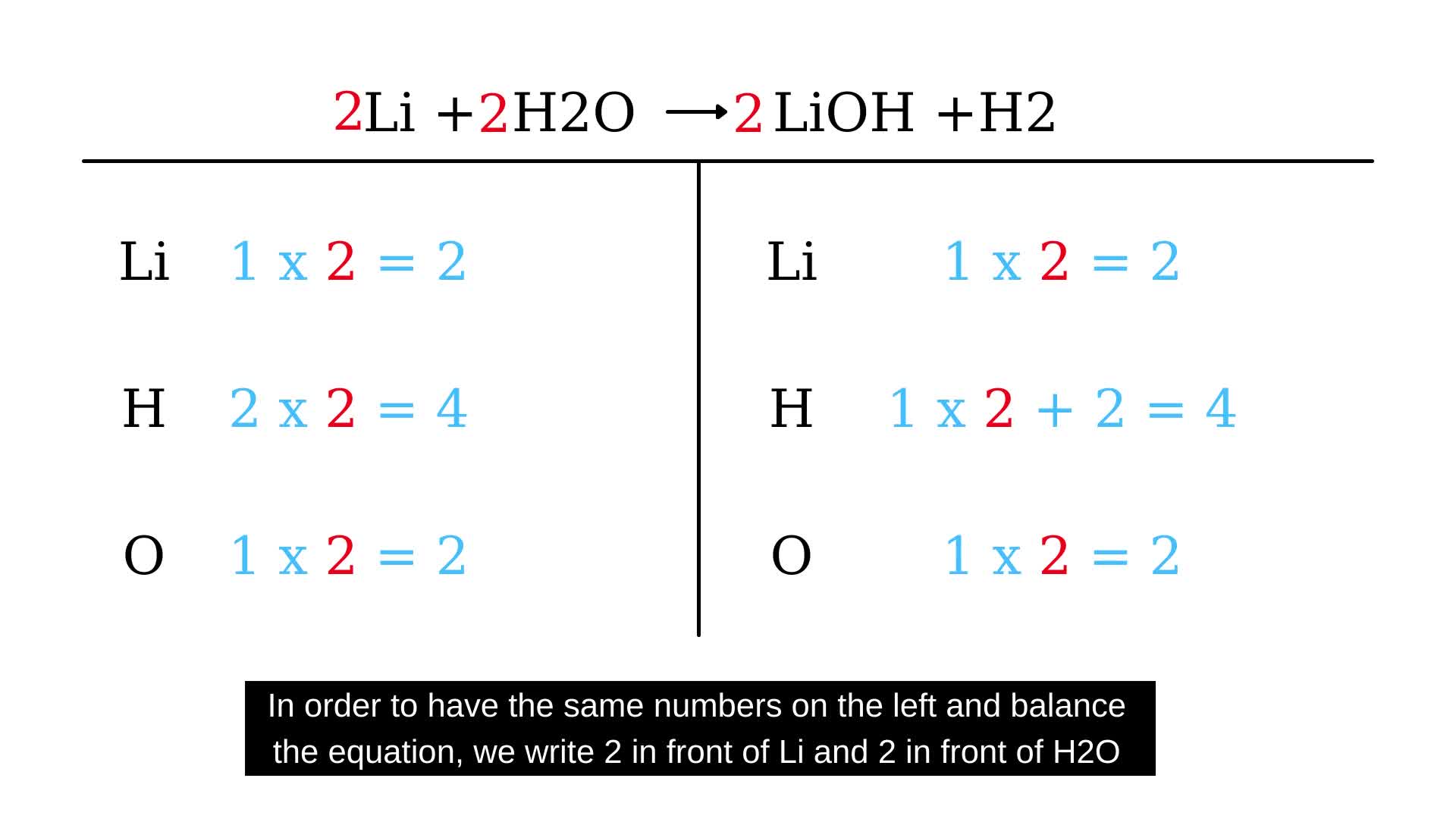
n mole each of h2o h2 o2 r tken in closed container at temperature t if y mole of h2 r disasssociated at equillibrium n equillibrium pressure is p the cgt66gee -Chemistry -

H2 Evolution from H2O via O–H Oxidative Addition Across a 9,10-Diboraanthracene | Inorganic Chemistry | ChemRxiv | Cambridge Open Engage

Reaction of CO, H2O, H2 and CO2 on the clean as well as O, OH and H precovered Fe(100) and Fe(111) surfaces - Catalysis Science & Technology (RSC Publishing)

![PDF] Two triple points in the H2O–H2 system† | Semantic Scholar PDF] Two triple points in the H2O–H2 system† | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/0e3a3f978107aae0e7b1ab47beca2a2ce22bd231/2-Figure1-1.png)

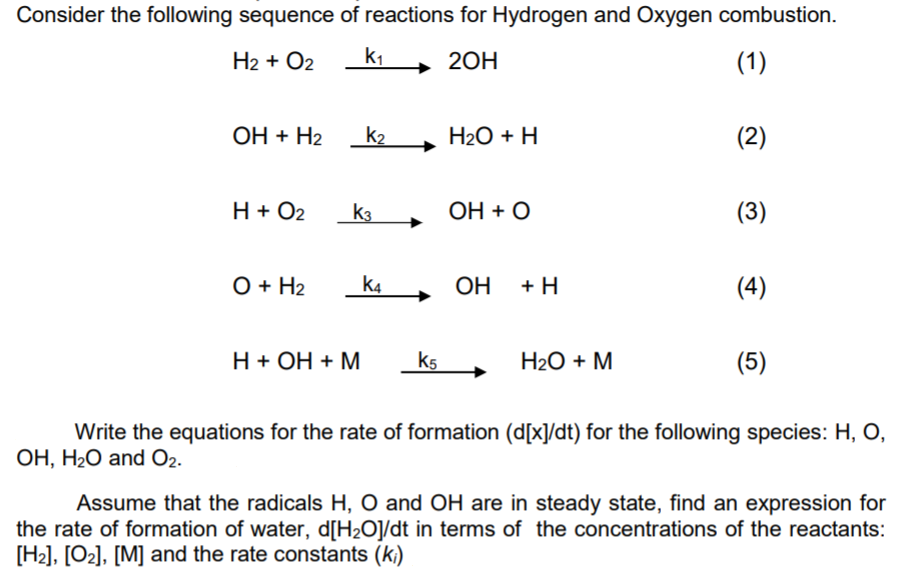
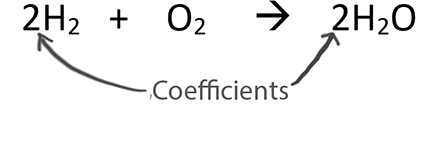
![PDF] Initiation in H2/O2: Rate constants for H2+O2→H+HO2 at high temperature | Semantic Scholar PDF] Initiation in H2/O2: Rate constants for H2+O2→H+HO2 at high temperature | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/59651c1a79d5be73d45e3b492f6f4396965dd05f/5-Table1-1.png)