![SOLVED:Iron(III) sulfate [Fe2(SO4)3] is composed of Fe3+ and SO4 2? ions. Explain why a sample of iron(III) sulfate is uncharged SOLVED:Iron(III) sulfate [Fe2(SO4)3] is composed of Fe3+ and SO4 2? ions. Explain why a sample of iron(III) sulfate is uncharged](https://cdn.numerade.com/previews/f4c581e9-32de-47c4-b1a9-adc9b9dcdcf8.gif)
SOLVED:Iron(III) sulfate [Fe2(SO4)3] is composed of Fe3+ and SO4 2? ions. Explain why a sample of iron(III) sulfate is uncharged

Stable positive electrolyte containing high-concentration Fe2(SO4)3 for vanadium flow battery at 50 °C - ScienceDirect

Monoclinic Fe2(SO4)3: A new Fe-based cathode material with superior electrochemical performances for Na-ion batteries - ScienceDirect
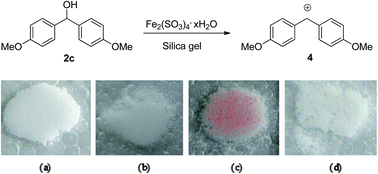
Fe2(SO4)3·xH2O on silica: an efficient and low-cost catalyst for the direct nucleophilic substitution of alcohols in solvent-free conditions - RSC Advances (RSC Publishing)



![PDF] Fe2(SO4)3·H2SO4·28H2O, a low-temperature water-rich iron(III) sulfate. | Semantic Scholar PDF] Fe2(SO4)3·H2SO4·28H2O, a low-temperature water-rich iron(III) sulfate. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/3a1ee865d6b22d384850851082185de0c92f6121/1-Figure1-1.png)