Experiment 8 - Precipitation of CaC2O4*H2O from the Salt Mixture Unknown name Moon Stardust Limiting - Studocu
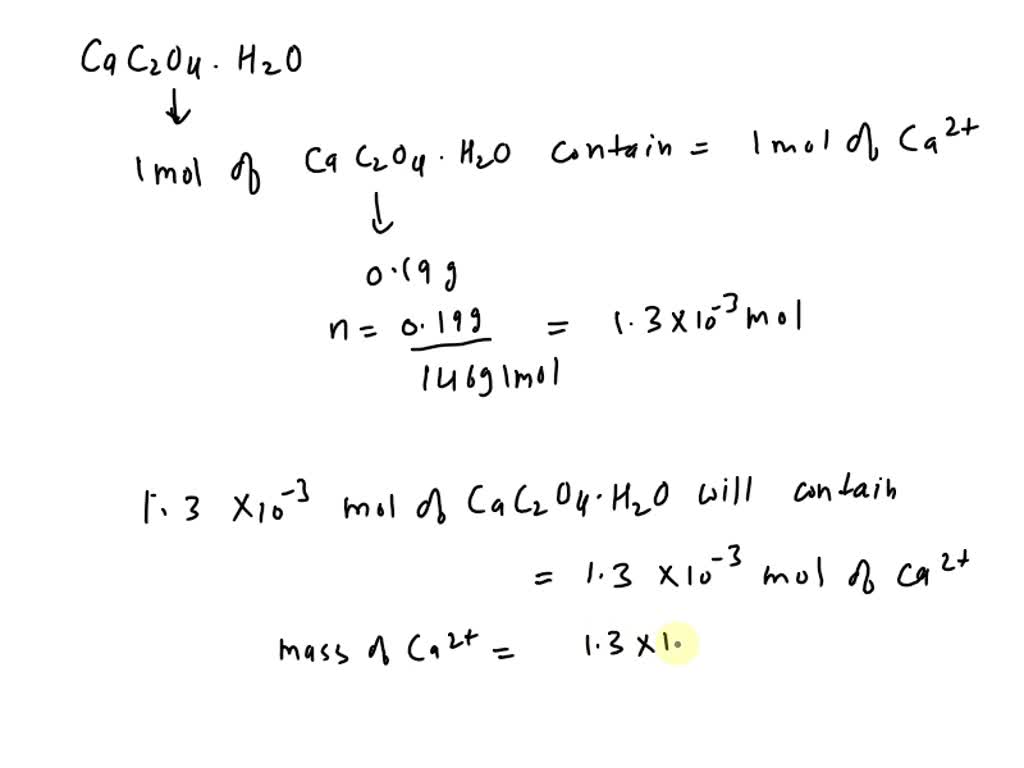
SOLVED: What is the concentration of calcium (Ca2+) in calcium oxalate monohydrate (CaC2O4.H2O). Ca2+(aq) + C2O42-(aq) + H2O (l) → CaC2O4·H2O (s) The mass of calcium oxalate monohydrate is 0.190grams. what is
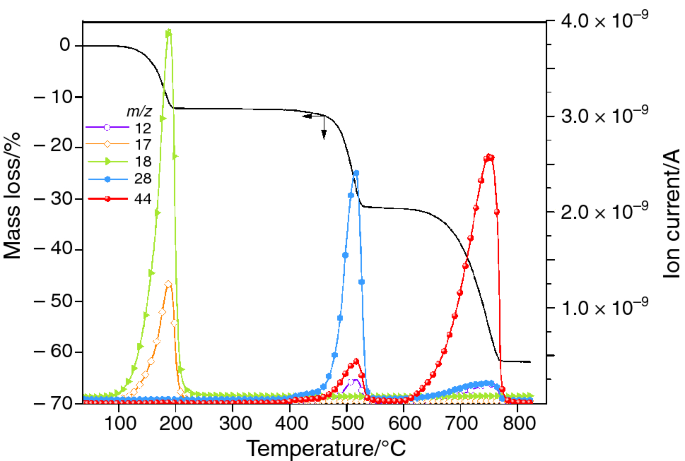
Thermal Stability of Calcium Oxalates from CO2 Sequestration for Storage Purposes: An In-Situ HT-XRPD and TGA Combined Study

Computational prediction of the pattern of thermal gravimetry data for the thermal decomposition of calcium oxalate monohydrate – topic of research paper in Chemical sciences. Download scholarly article PDF and read for
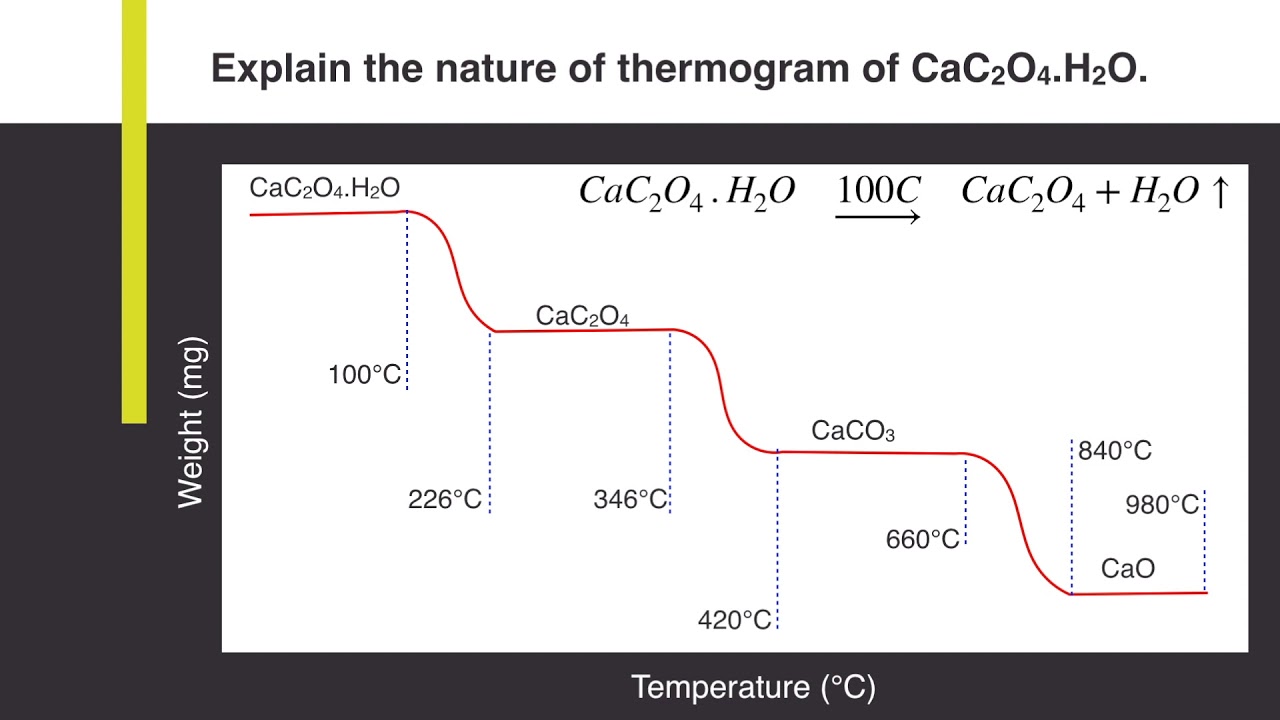
Explain the nature of thermogram of Calcium Oxalate Monohydrate (CaC2O4.H2O) | Analytical Chemistry - YouTube
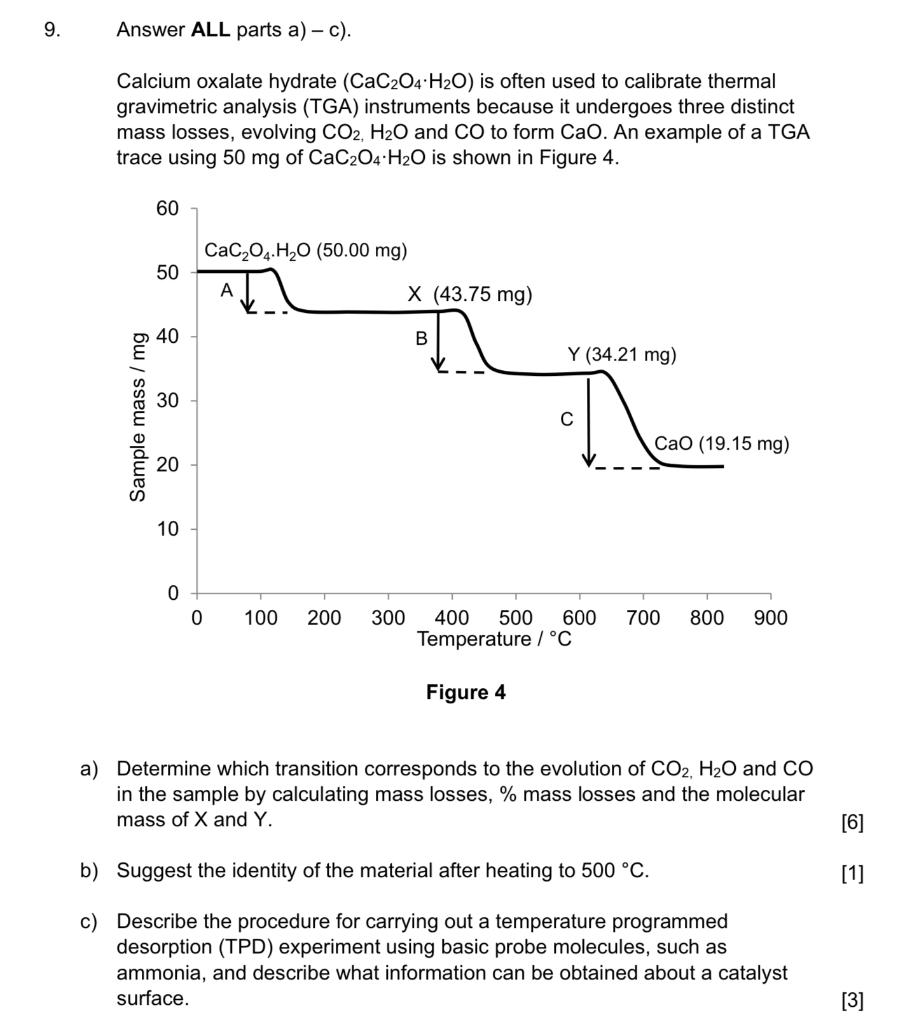
1 Chapter 3 The principle of TGA and its applications: TGA is thermogravimetric analysis. It is one of the thermal method of an
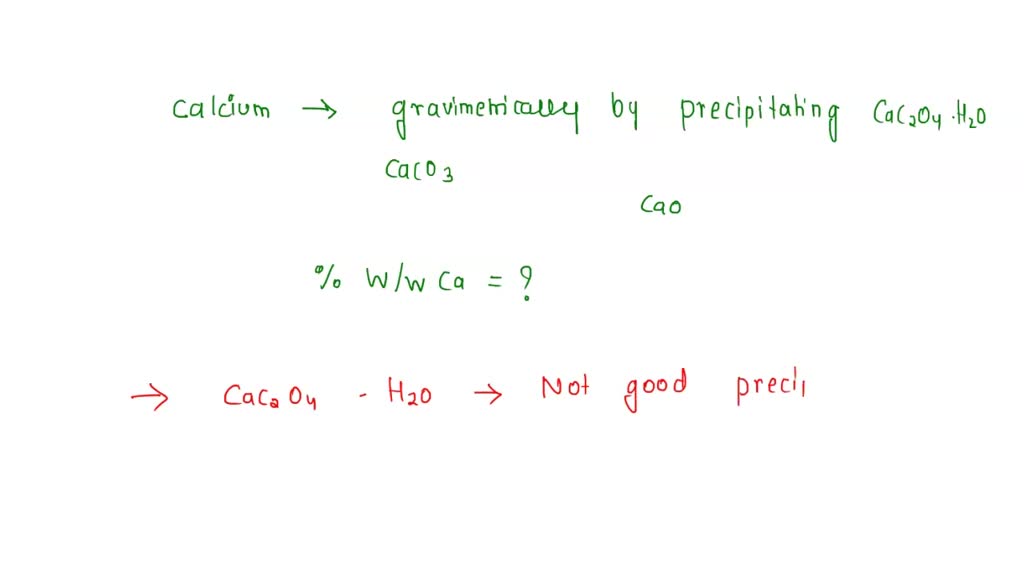
SOLVED: Calcium is determined gravimetrically by precipitating CaC2O4•H2O and isolating CaCO3. After dissolving a sample in 10 mL of water and 15 mL of 6 M HCl, the resulting solution is heated

Solubility of Calcium Oxalate Monohydrate in Concentrated Electrolyte Solutions | Journal of Chemical & Engineering Data
Calcium oxalate, CaC2O4.H2O, is a sparingly soluble salt of analytical and physiological importance. - Sarthaks eConnect | Largest Online Education Community
1 Chapter 3 The principle of TGA and its applications: TGA is thermogravimetric analysis. It is one of the thermal method of an