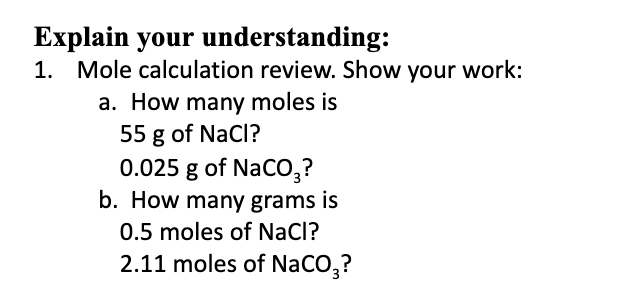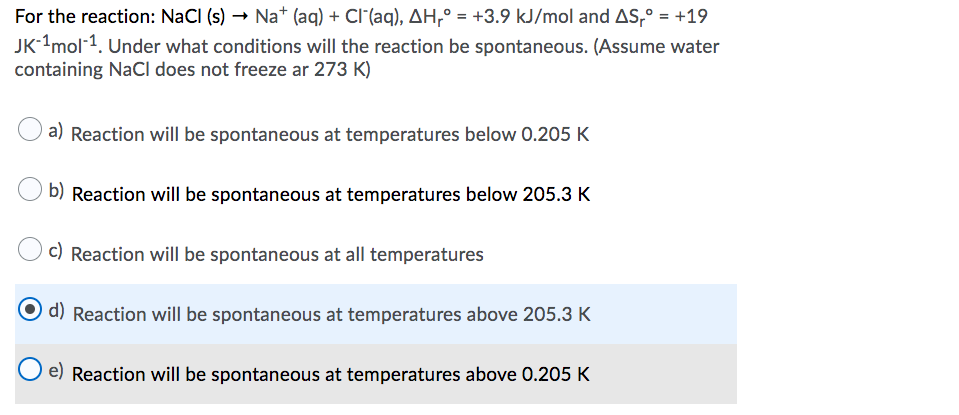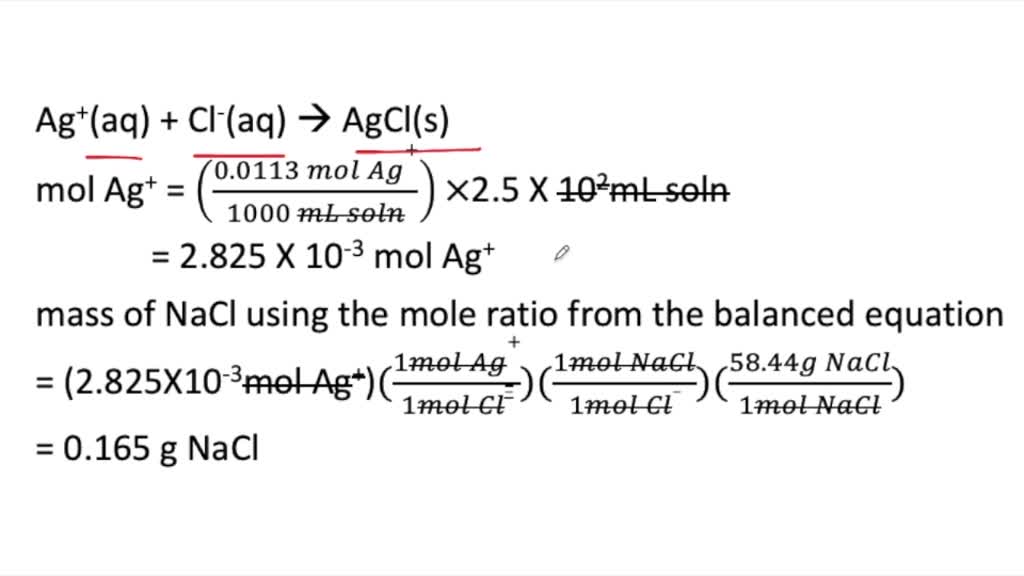
SOLVED:How many grams of NaCl are required to precipitate most of the Ag ions from 2.50 ×10^2 mL of a 0.0113 M AgNO3 solution? Write the net ionic equation for the reaction.

Explain why on addition of 1 mol of NaCl to 1 litre of water, the boiling point of water increases, while addition of 1 mol of methyl alcohol to one litre of

3/4/2016 I ObjectiveDo Now Convert grams of a substance to moles of a substance. Calculate the molar mass of: NaCl MgCl ppt download

Molarity Problems – IF worksheet. 1)MW NaCl = 23 + 35 = 58g NaCl = 1 mole 2)M = moles M = x moles Liters 1.0 L 3)58 g NaCl 1 mole NaCl = 1 mole NaCl 58. - ppt download

SEM images of the samples electro-deoxidized in the CaCl 2-NaCl-1mol%... | Download Scientific Diagram

Concentration Calculations Molarity. Objectives To calculate the molecular weight and moles of a substance To calculate the Molarity of a substance using. - ppt download

XRD pattern of the sample immersed in the CaCl 2-NaCl-1mol% CaO melt... | Download Scientific Diagram